Biomedical Device Testing
ISO 18562-3 VOS Emissions Testing for Biomedical Device Breathing Gas Pathways
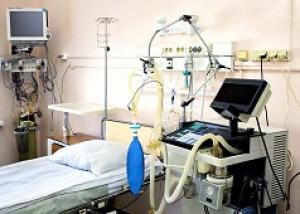 Supporting Biocompatibility Assessments and Regulatory Compliance
Supporting Biocompatibility Assessments and Regulatory Compliance
The ISO 18562 standards require a biomedical respiratory device that delivers inhalation gas to patients to undergo emissions testing. Testing is followed by a biocompatibility evaluation as part of a structured evaluation program within a risk management framework. This evaluation program is required to encompass the expected service life of the device from first use to end of life.
For respiratory devices, volatile organic substances are typically the most important chemical class because they can result in unsafe inhalation exposures. The 2024 version of ISO 18562-3, which FDA requires for premarket conformity declarations starting July 2026, provides the guidelines for measuring the emissions of volatile organic substances (VOS) — historically referred to as volatile organic compounds (VOCs) — emitted from breathing gas pathways. These VOS emissions mostly derive from the diffusion of organic chemicals from the various polymeric materials used in gas pathways. Patient exposures to particulate matter (PM) may also be of concern. If so, ISO 18562-2 is used to measure PM emissions. ISO 18562-1 defines the requirements for the biocompatibility assessment of the results generated by the tests.
Berkeley Analytical is an expert laboratory with 30+ years of experience in the measurement of organic chemical emissions from many types of polymeric materials. Building upon this foundation, we provide ISO 18562-3:2024 testing of biomedical devices, accessories, components, and materials to support research and development, biocompatibility assessments, and FDA 510(k) submissions. We also provide ISO 18562-2:2024 testing of PM. Our biomedical device testing services are summarized in a downloadable brochure.
Our goals in this field are straightforward – to generate clear, defensible emissions data that stands up to regulatory scrutiny in a timely manner.
Our Testing Approach
ISO 18562 testing involves controlled simulation of device use, careful chemical analysis, and detailed reporting that aligns with regulatory expectations.
Realistic Use Simulation
Devices and accessories are evaluated in environmental chamber facilities designed specifically for trace-level emissions testing.
With these facilities, we replicate:
- Published operating conditions
- Maximum published temperatures when required
- Patient category appropriate airflow rates
Trace-Level Chemical Analysis
Volatile organic substances are collected using sorbent-based sampling media and analyzed by thermal desorption gas chromatography/mass spectrometry (TD-GC/MS) in accordance with ISO 16000-6.
Our testing provides:
- Characterization of very volatile, volatile, and semi-volatile compounds
- Identification of emitted compounds
- Quantification of these compounds at low microgram-per-cubic-meter concentrations, typically 1 to 2 µg/m3
Where required, we also perform aldehyde analysis by ISO 16000-3 and particulate matter emissions testing by ISO 18562-2 using gravimetric and particle-counting methods.
Reporting Designed for Regulatory Review
Clear reporting is critical. Our emissions reports are structured to support:
- Review of VOS emission profiles
- Exposure estimations based on device use
- Biocompatibility assessments with respect to applicable toxicological thresholds
- FDA submissions
When appropriate, we coordinate with an experienced board-certified toxicologist to support the profile reviews and biocompatibility assessments.
Our Laboratory Infrastructure
Our Richmond, California laboratory is well equipped with environmental chamber facilities and analytical instrumentation. We operate:
- More than 20 ultra-low background environmental chambers
- Four mid-scale chambers for full-device evaluations
- Micro-scale chamber systems for material R&D and component screening
- Multiple automated thermal desorption GC/MS systems for trace level chemical analysis
These facilities allow manufacturers to use the same laboratory partner from early product development through final compliance testing of commercial devices.
Devices and Materials We Support
We perform ISO 18562-3 and ISO 18562-2 testing for a broad range of products and materials, including:
- Active respiratory devices such as ventilators, CPAP and BiPAP systems, oxygen concentrators, and oxygen conserving regulators
- Breathing gas pathway accessories including tubing, masks, connectors, and breathing circuits
- Breathing gas pathway components and materials such as, adhesives, elastomers, lubricants, and filter media
Why Manufacturers Choose to Work With Berkeley Analytical
Access – Our customers work directly with a scientific manager who oversees the test program from the planning stage through final reporting. We focus on practical test design, predictable timelines, and data packages structured for regulatory use.
Fast turnaround – Most ISO 18562 testing programs are completed in approximately 30 days.
Regulatory-Focused Reporting – We provide clear, detailed data reports that withstand FDA scrutiny.
Begin Your ISO 18562 Testing Program
If you require regulatory compliance testing of a biomedical respiratory device or an accessory for VOCs and PM emissions, we’d like to hear from you. Please send us an approved testing plan, and we’ll respond with a quote.
If you’re developing or modifying an existing breathing gas pathway device, or trying to qualify suppliers and replacement parts, we can design a screening test program to help ensure that the materials and components you select will have acceptably low volatile organic emissions.
We also have experience with end-of-life product tests, now frequently required by the FDA.
Contact Berkeley Analytical to discuss your project.
Berkeley Analytical
Richmond, California | Serving Clients Globally
Phone: +1 (510) 236-2325
Email: info@berkeleyanalytical.com

